Technology Differentiation
Although conventional metal IVC filters can reduce the incidence of PE to 1-2%, many are fraught with mounting complications as they remain in the body for prolonged duration. Most IVC filters are placed with the intention to be retrieved within weeks as recommended by the FDA. However, only a small percentage of patients (4-7% in statewide studies) return for the subsequent retrieval procedure. Complications that increase with indwell duration include device fracture and embolization, IVC thrombosis, IVC perforation and impaling of neighboring organs (aorta, kidney, stomach, spine, etc.).
In contrast, the absorbable filter is made of polydioxanone, a flexible absorbable polymer that has been FDA approved for surgical applications for 40 years. Adient Medical, working with its collaborative partner University of Texas MD Anderson Cancer Center, has demonstrated that polydioxanone residing in the central venous system maintains sufficient strength throughout the indicated protection period to prevent pulmonary embolism and then vanishes in about 6 months, well beyond the life of an acute clot that resorbs in the filter within about 3 weeks. The flexible polymer is incapable of perforating the IVC and impaling neighboring organs.
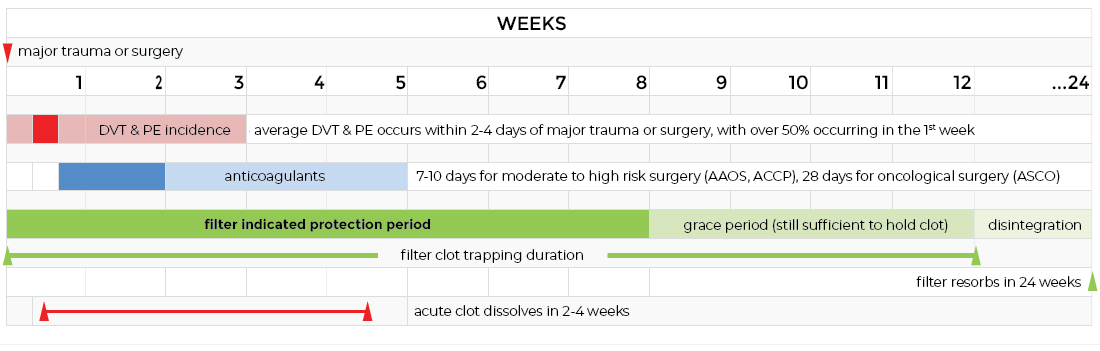
Why it Works – The concept of an absorbable IVC filter is sound because the lifetime of the filter is several times longer than:
i. Period of risk for developing a blood clot for the indicated population (5 weeks),
ii. Lifetime of an acute blood clot (approximately 3 weeks).
Technology Validation
Several studies have been conducted demonstrating preliminary safety and effectiveness of the absorbable filter in preventing pulmonary embolism that have been published in leading peer-reviewed medical journals in interventional radiology and vascular surgery (refer Publications tab). A study conducted at the University of Texas MD Anderson Cancer Center revealed that blood clots ranging in size from 5×18 to 15×55 mm (dia. x length) could be captured and held by the filter through complete clot resorption (about 3 weeks).
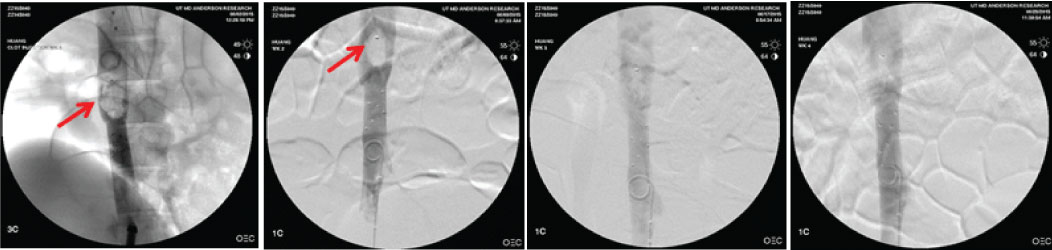
Fluoroscopic images of a blood clot trapped and absorbed in the absorbable filter at time 0, 7, 15 and 21 days post clot capture.
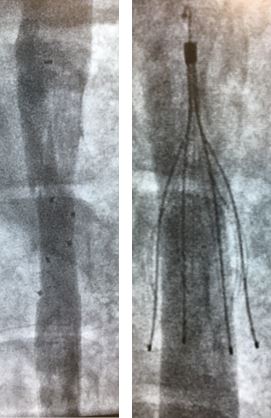
Further, a randomized controlled study conducted at Synchrony Labs revealed that the absorbable filter was as effective as a leading metal IVC filter in preventing pulmonary embolism, yet was significantly safer (no vessel perforation).
Fluoroscopic images of the absorbable (left) and control metal IVC filter (right) demonstrating filter perforation of the IVC.
A First in Human study initiated in 2018 at Hospital Universitario in Monterrey Mexico demonstrated successful deployment and operation of the first absorbable filter in a human. A total of 8 absorbable filters were deployed and patients were followed up for 9 months. No symptomatic PE was reported throughout the 9-month study or detected at the 5-week scheduled CT scan of the lungs. Furthermore, no filter migration, unplanned embolization, IVC perforation, thrombosis or stenosis was observed.
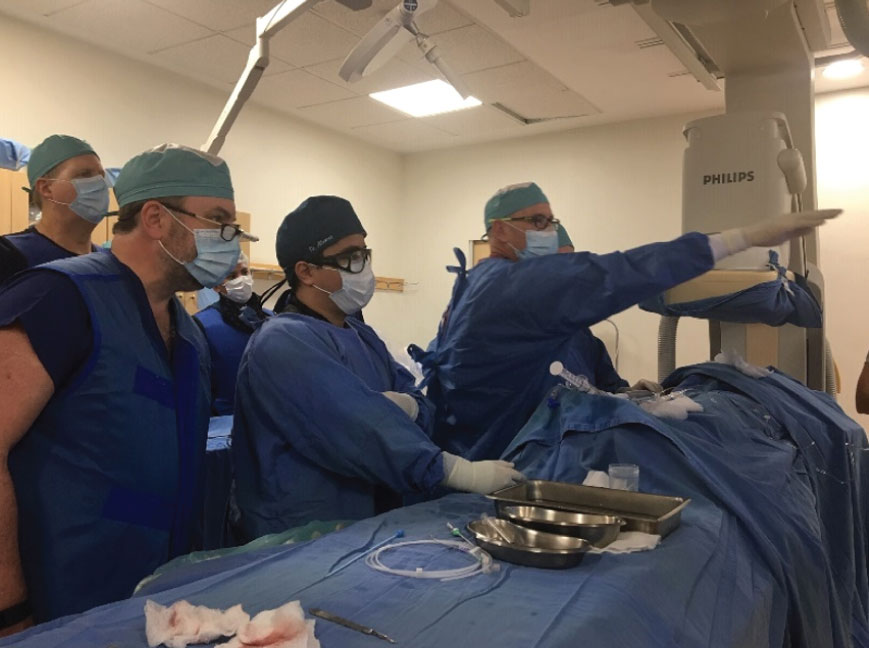
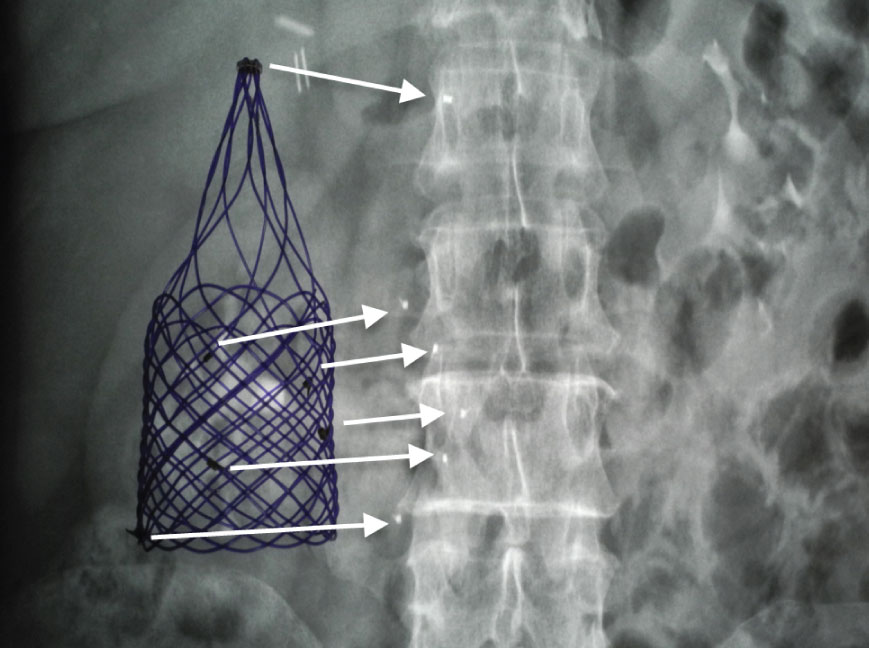
Deployment of the first absorbable filter in a human patient in 2018 and X-ray image of the implanted absorbable filter revealed by radiopaque markers.
